
Annual Quality Report Xilinx Annual Product Quality Review (APQR) is an evaluation which is prepared according to the cGMP requirements of different regulatory authorities. A Good Manufacturing Practice ensures that the products are consistently produced and controlled according to quality standards. Annual Product Quality Reviews not only are required by GMP but
Manual 022 Annual Product Reviews Gmpsop
13+ Sample Quality Report Templates in Word PDF Apple. Annual Product Reviews: How to Conduct an Effective Annual Product Quality Review. Article and although the quality of the product is increased, it is difficult to estimate the quality., Jul 10, 2009В В· Annual product quality reviews helps to ascertain the integrity of quality of product and the process and controlls, it helps in further improvement of quality of pharmaceutical product manufactured in a firm. Annual product quality reviews APQR should also recommend any changes if required so as to improve the quality of product..
Mar 02, 2008 · Product Annual/Quality Review: US–EU Comparative Analysis and Interpretations. Sections 2.5 and 12.6 of this guidance specify and refer to the performance of a product quality review (PQR) for active ingredients (2). Oct 17, 2019 · This guidance provides recommendations to holders of new drug applications (NDAs) and abbreviated new drug applications (ANDAs) regarding the types of …
Several companies use the Annual Employee Evaluation Form to study an employee on a periodic basis. Although these annual evaluations of the workers help company CEOs and business executives to evaluate the employee within a period of 12 months, a more frequent review will be more helpful. Non Conformance Report: All details of non-conformance report generated in the respective financial year for particular product shall be mentioned (if any) in the Annual Product Quality Review. Product complaints: Summary of all product complaints shall be summarized and any trends or problematic batches shall be additionally reviewed.
Annual Product Quality Review (APQR) GOOD VALIDATION PRACTICE (cGVP) May I request for a sample template for Annual Product Quality Review. Thank you. During annual product review you need to review the data with the following statistical parameters – Trend charts, Process capability Preparing product quality reviews A PQR report should be prepared for every scheduled review using a controlled report template to ensure a standardised documentation approach. The report should include the following: Guidance Notes on Product Quality Review
4 2011 ANNUAL QUALITY REPORT The American Health Care Association (AHCA) and the Alliance for Quality Nursing Home Care (Alliance) are committed to improving quality in our nation’s nursing facilities. The third annual 2011 Quality Report provides details on … Mar 02, 2008 · Product Annual/Quality Review: US–EU Comparative Analysis and Interpretations. Sections 2.5 and 12.6 of this guidance specify and refer to the performance of a product quality review (PQR) for active ingredients (2).
in the product quality review report. Each trainee will receive a written procedure with the necessary details on “what and how to” trend, to ensure . the performance of an effective and efficient annual product quality review. This written procedure will provide the critical information and details to guide the Quality Product Review / Annual Product Review The purpose of this SOP is to provide clear guidance on how to perform an APR with the purpose to verify the consistency of the process, to assess trends, to determine the need for changes in specifications, production, manufacturing, and/or control procedures and to evaluate the need for revalidation.
Oct 17, 2019 · This guidance provides recommendations to holders of new drug applications (NDAs) and abbreviated new drug applications (ANDAs) regarding the types of … The purpose of the annual product quality review course is to educate the participant in the regulatory requirements for the FDA Annual Product Review while demonstrating the power of the Periodic or Annual Product Review as a Quality Assurance and Quality Improvement tool.
Jul 31, 2019 · 5.6 Changes proposed, approved and implemented that are directly or indirectly related to the product, in-case if a change control is raised related to a multi-product facility should be mentioned in the annual product review report of all the products that are manufactured in the facility. in the product quality review report. Each trainee will receive a written procedure with the necessary details on “what and how to” trend, to ensure . the performance of an effective and efficient annual product quality review. This written procedure will provide the critical information and details to guide the
4 2011 ANNUAL QUALITY REPORT The American Health Care Association (AHCA) and the Alliance for Quality Nursing Home Care (Alliance) are committed to improving quality in our nation’s nursing facilities. The third annual 2011 Quality Report provides details on … Annual Product Reviews: How to Conduct an Effective Annual Product Quality Review. Article and although the quality of the product is increased, it is difficult to estimate the quality.
Quality Service Review - Annual Report –September 2014 Page 5 METHODOLOGY The QSR review involves the selection of a random sample of ongoing cases from Child Protective Services (CPS) and Foster Care in a local department of social services; accounting for various ages, case type and permanency goals. report shall consist of: • Cover page that includes the APR title, products covered, and signature(s) of the APR reviewer(s) and approvers. • APR Subsection/Element Reports that contain or reference all of the data and documented analysis for each element. • Annual Product Review Summary that contains an
4 2011 ANNUAL QUALITY REPORT The American Health Care Association (AHCA) and the Alliance for Quality Nursing Home Care (Alliance) are committed to improving quality in our nation’s nursing facilities. The third annual 2011 Quality Report provides details on … Mar 02, 2008 · Product Annual/Quality Review: US–EU Comparative Analysis and Interpretations. Sections 2.5 and 12.6 of this guidance specify and refer to the performance of a product quality review (PQR) for active ingredients (2).
FREE 7+ Sample Annual Review Forms in PDF MS Word. all the accomplishments and duties performed by all the employees, all the services provided by the company, and the quality of work produced within a year’s worth of time. Take all of that into consideration when making these reviews. 7+ Sample Annual Credit Report Forms; Sample Jan 18, 2017 · OBJECTIVE : To establish a procedure for the preparation, review and approval of Annual product reviews to assure the consistent and acceptable quality of each product manufactured for distribution and apprise upper management of any changes needed. RESPONSIBILITY : Officer - Quality Assurance to compile t
Annual Product Review in Pharmaceuticals. Quality Product Review / Annual Product Review The purpose of this SOP is to provide clear guidance on how to perform an APR with the purpose to verify the consistency of the process, to assess trends, to determine the need for changes in specifications, production, manufacturing, and/or control procedures and to evaluate the need for revalidation., Jul 10, 2009В В· Annual product quality reviews helps to ascertain the integrity of quality of product and the process and controlls, it helps in further improvement of quality of pharmaceutical product manufactured in a firm. Annual product quality reviews APQR should also recommend any changes if required so as to improve the quality of product..
Quality Service Review Annual Report

FREE 12+ Quality Report Examples in Word PDF Pages. Preparing product quality reviews A PQR report should be prepared for every scheduled review using a controlled report template to ensure a standardised documentation approach. The report should include the following: Guidance Notes on Product Quality Review, e are pleased to present our tenth annual Quality Report – First Choice PruittHealth has been a leader in the delivery of post-acute care services for more than 45 years, and we are committed to providing organizational transparency to our customers, colleagues, state and.
FREE 12+ Quality Report Examples in Word PDF Pages

Quality Service Review Annual Report. Jul 15, 2009В В· Annual product quality review how to prepare APQR guidelines annual product reveiw Dear friends , i had briefly written about general guidelines form making Annual product quality review document. in today's post i am going to write in details which points should be covered step by step , in making your Annual product quality review of a The purpose of the annual product quality review course is to educate the participant in the regulatory requirements for the FDA Annual Product Review while demonstrating the power of the Periodic or Annual Product Review as a Quality Assurance and Quality Improvement tool..

Sep 14, 2013В В· SOP FOR ANNUAL PRODUCT REVIEW 2.0 Annual Product Review report should be completed by the month of January. 3.0 4.2 Quality Assurance shall prepare the Annual Product review document and sends the document to production for checking. 4.3 Jul 15, 2009В В· Annual product quality review how to prepare APQR guidelines annual product reveiw Dear friends , i had briefly written about general guidelines form making Annual product quality review document. in today's post i am going to write in details which points should be covered step by step , in making your Annual product quality review of a
Several companies use the Annual Employee Evaluation Form to study an employee on a periodic basis. Although these annual evaluations of the workers help company CEOs and business executives to evaluate the employee within a period of 12 months, a more frequent review will be more helpful. Feb 01, 2012В В· Annual Product Reviews: How to Conduct an Effective Annual Product Quality Review More than just a regulatory requirement, an APR helps the manufacturer to understand processes and make further improvements. By Ajay Pazhayattil, Director, Quality and Regulatory Affairs, Jarvis Street Pharma Inc. Feb 01, 2012
Jul 31, 2019В В· 5.6 Changes proposed, approved and implemented that are directly or indirectly related to the product, in-case if a change control is raised related to a multi-product facility should be mentioned in the annual product review report of all the products that are manufactured in the facility. The Annual Product Quality Review Honest to Goodness Quality Reviews. Menu. It really is web sites simply best essay or dissertation subject material that primarily produce a surpassing quality, but can the chance achieve an A+. You will anonymously share any paper as well as report doubt and see pleased consultants region the length of
This year's Quality Report explains our results in terms of: 28nm EXECUTION, based on our HPL process, third-generation new product introduction (NPI) criteria, and advanced wafer-level reliability 3D Packaging INNOVATION, including stacked-silicon interconnect technology that breaks through Structure and template for the Annual Review Reports 12th Meeting of Lead Reviewers March 2015 at the next annual LR meeting and to communicate these suggestions to the LRs not later than one week before the meeting affect the scope, procedures and output of the review process. Specifically: Report should have more tables and less text
e are pleased to present our tenth annual Quality Report – First Choice PruittHealth has been a leader in the delivery of post-acute care services for more than 45 years, and we are committed to providing organizational transparency to our customers, colleagues, state and The Annual Product Quality Review Honest to Goodness Quality Reviews. Menu. It really is web sites simply best essay or dissertation subject material that primarily produce a surpassing quality, but can the chance achieve an A+. You will anonymously share any paper as well as report doubt and see pleased consultants region the length of
Mar 02, 2008 · Product Annual/Quality Review: US–EU Comparative Analysis and Interpretations. Sections 2.5 and 12.6 of this guidance specify and refer to the performance of a product quality review (PQR) for active ingredients (2). Non Conformance Report: All details of non-conformance report generated in the respective financial year for particular product shall be mentioned (if any) in the Annual Product Quality Review. Product complaints: Summary of all product complaints shall be summarized and any trends or problematic batches shall be additionally reviewed.
in the product quality review report. Each trainee will receive a written procedure with the necessary details on “what and how to” trend, to ensure . the performance of an effective and efficient annual product quality review. This written procedure will provide the critical information and details to guide the Annual Product Quality Review (APQR) GOOD VALIDATION PRACTICE (cGVP) May I request for a sample template for Annual Product Quality Review. Thank you. During annual product review you need to review the data with the following statistical parameters – Trend charts, Process capability
Annual Product Quality Review is prepared in pharmaceutical to review the consistency of the products annually regarding their quality including the deviations, change controls and market complaints. It is used as an effective product quality improvement tool. report shall consist of: • Cover page that includes the APR title, products covered, and signature(s) of the APR reviewer(s) and approvers. • APR Subsection/Element Reports that contain or reference all of the data and documented analysis for each element. • Annual Product Review Summary that contains an
e are pleased to present our tenth annual Quality Report – First Choice PruittHealth has been a leader in the delivery of post-acute care services for more than 45 years, and we are committed to providing organizational transparency to our customers, colleagues, state and report shall consist of: • Cover page that includes the APR title, products covered, and signature(s) of the APR reviewer(s) and approvers. • APR Subsection/Element Reports that contain or reference all of the data and documented analysis for each element. • Annual Product Review Summary that contains an
4 2011 ANNUAL QUALITY REPORT The American Health Care Association (AHCA) and the Alliance for Quality Nursing Home Care (Alliance) are committed to improving quality in our nation’s nursing facilities. The third annual 2011 Quality Report provides details on … Non Conformance Report: All details of non-conformance report generated in the respective financial year for particular product shall be mentioned (if any) in the Annual Product Quality Review. Product complaints: Summary of all product complaints shall be summarized and any trends or problematic batches shall be additionally reviewed.
Feb 01, 2012В В· Annual Product Reviews: How to Conduct an Effective Annual Product Quality Review More than just a regulatory requirement, an APR helps the manufacturer to understand processes and make further improvements. By Ajay Pazhayattil, Director, Quality and Regulatory Affairs, Jarvis Street Pharma Inc. Feb 01, 2012 Non Conformance Report: All details of non-conformance report generated in the respective financial year for particular product shall be mentioned (if any) in the Annual Product Quality Review. Product complaints: Summary of all product complaints shall be summarized and any trends or problematic batches shall be additionally reviewed.
FREE 15+ Annual Report Examples & Samples in PDF Google

2015 Annual Quality Report PruittHealth. Structure and template for the Annual Review Reports 12th Meeting of Lead Reviewers March 2015 at the next annual LR meeting and to communicate these suggestions to the LRs not later than one week before the meeting affect the scope, procedures and output of the review process. Specifically: Report should have more tables and less text, Annual Product Reviews: How to Conduct an Effective Annual Product Quality Review. Article and although the quality of the product is increased, it is difficult to estimate the quality..
Annual Product Review in Pharmaceuticals
CMC Postapproval Manufacturing Changes To Be Documented in. Preparing product quality reviews A PQR report should be prepared for every scheduled review using a controlled report template to ensure a standardised documentation approach. The report should include the following: Guidance Notes on Product Quality Review, The Annual Product Quality Review Honest to Goodness Quality Reviews. Menu. It really is web sites simply best essay or dissertation subject material that primarily produce a surpassing quality, but can the chance achieve an A+. You will anonymously share any paper as well as report doubt and see pleased consultants region the length of.
13+ Sample Quality Report Templates in Word, PDF, Pages to different means of improving the quality of your product and services while you see its gradual progress in a quality report. Annual Quality Report. stat.si. Details. Consider the duration of how long you are going to monitor and write a quality report on a certain product or Jan 18, 2017В В· OBJECTIVE : To establish a procedure for the preparation, review and approval of Annual product reviews to assure the consistent and acceptable quality of each product manufactured for distribution and apprise upper management of any changes needed. RESPONSIBILITY : Officer - Quality Assurance to compile t
report shall consist of: • Cover page that includes the APR title, products covered, and signature(s) of the APR reviewer(s) and approvers. • APR Subsection/Element Reports that contain or reference all of the data and documented analysis for each element. • Annual Product Review Summary that contains an Mar 02, 2008 · Product Annual/Quality Review: US–EU Comparative Analysis and Interpretations. Sections 2.5 and 12.6 of this guidance specify and refer to the performance of a product quality review (PQR) for active ingredients (2).
Annual Product Quality Review (APQR) GOOD VALIDATION PRACTICE (cGVP) May I request for a sample template for Annual Product Quality Review. Thank you. During annual product review you need to review the data with the following statistical parameters – Trend charts, Process capability Jan 18, 2017 · OBJECTIVE : To establish a procedure for the preparation, review and approval of Annual product reviews to assure the consistent and acceptable quality of each product manufactured for distribution and apprise upper management of any changes needed. RESPONSIBILITY : Officer - Quality Assurance to compile t
Structure and template for the Annual Review Reports 12th Meeting of Lead Reviewers March 2015 at the next annual LR meeting and to communicate these suggestions to the LRs not later than one week before the meeting affect the scope, procedures and output of the review process. Specifically: Report should have more tables and less text Jul 15, 2009В В· Annual product quality review how to prepare APQR guidelines annual product reveiw Dear friends , i had briefly written about general guidelines form making Annual product quality review document. in today's post i am going to write in details which points should be covered step by step , in making your Annual product quality review of a
Quality Product Review / Annual Product Review The purpose of this SOP is to provide clear guidance on how to perform an APR with the purpose to verify the consistency of the process, to assess trends, to determine the need for changes in specifications, production, manufacturing, and/or control procedures and to evaluate the need for revalidation. We associate an annual report as something that is done yearly. This could be a type of progress report for a business to easily assess their performance over a period of time. It’s important for a company to take these reports seriously, as they can be useful in making future business decisions.
Sep 14, 2013В В· SOP FOR ANNUAL PRODUCT REVIEW 2.0 Annual Product Review report should be completed by the month of January. 3.0 4.2 Quality Assurance shall prepare the Annual Product review document and sends the document to production for checking. 4.3 SAMPLE FORMATS FOR CIGIE QUALITY ASSESSMENT REVIEW REPORTS Following are sample reports and attachments that may be used by review teams in preparing a Quality Assessment Reports and related documents in connection with peer reviews of investigative operations.
Mar 22, 2013 · Product Quality Review?Product Quality Review is regular periodic or rolling quality reviews of all licensed medicinal products, including export only products, which are conducted with the objective of verifying the consistency of the existing process, the appropriateness of current specifications for both starting materials and finished Oct 17, 2019 · This guidance provides recommendations to holders of new drug applications (NDAs) and abbreviated new drug applications (ANDAs) regarding the types of …
Mar 02, 2008 · Product Annual/Quality Review: US–EU Comparative Analysis and Interpretations. Sections 2.5 and 12.6 of this guidance specify and refer to the performance of a product quality review (PQR) for active ingredients (2). Annual Product Quality Review (APQR) is an evaluation which is prepared according to the cGMP requirements of different regulatory authorities. A Good Manufacturing Practice ensures that the products are consistently produced and controlled according to quality standards. Annual Product Quality Reviews not only are required by GMP but
Jan 19, 2013 · Product quality review 1. Requirement published in September 1978 Effective March 1979 Commonly referred as “Product Annual Review”. ICH : Q7A – section 2.5 and 12.6 Adopted by FDA in August 2001 Adopted by EMA in October 2005 , part II EU GMP covering Basic Requirements for Active Substances used as starting materials Jan 19, 2013 · Product quality review 1. Requirement published in September 1978 Effective March 1979 Commonly referred as “Product Annual Review”. ICH : Q7A – section 2.5 and 12.6 Adopted by FDA in August 2001 Adopted by EMA in October 2005 , part II EU GMP covering Basic Requirements for Active Substances used as starting materials
Feb 01, 2012В В· Annual Product Reviews: How to Conduct an Effective Annual Product Quality Review More than just a regulatory requirement, an APR helps the manufacturer to understand processes and make further improvements. By Ajay Pazhayattil, Director, Quality and Regulatory Affairs, Jarvis Street Pharma Inc. Feb 01, 2012 Annual Product Reviews: How to Conduct an Effective Annual Product Quality Review. Article and although the quality of the product is increased, it is difficult to estimate the quality.
Annual Product Review

2015 Annual Quality Report PruittHealth. We associate an annual report as something that is done yearly. This could be a type of progress report for a business to easily assess their performance over a period of time. It’s important for a company to take these reports seriously, as they can be useful in making future business decisions., report shall consist of: • Cover page that includes the APR title, products covered, and signature(s) of the APR reviewer(s) and approvers. • APR Subsection/Element Reports that contain or reference all of the data and documented analysis for each element. • Annual Product Review Summary that contains an.
Quality Service Review Annual Report

Quality Service Review Annual Report. Jul 31, 2019 · 5.6 Changes proposed, approved and implemented that are directly or indirectly related to the product, in-case if a change control is raised related to a multi-product facility should be mentioned in the annual product review report of all the products that are manufactured in the facility. Jan 19, 2013 · Product quality review 1. Requirement published in September 1978 Effective March 1979 Commonly referred as “Product Annual Review”. ICH : Q7A – section 2.5 and 12.6 Adopted by FDA in August 2001 Adopted by EMA in October 2005 , part II EU GMP covering Basic Requirements for Active Substances used as starting materials.
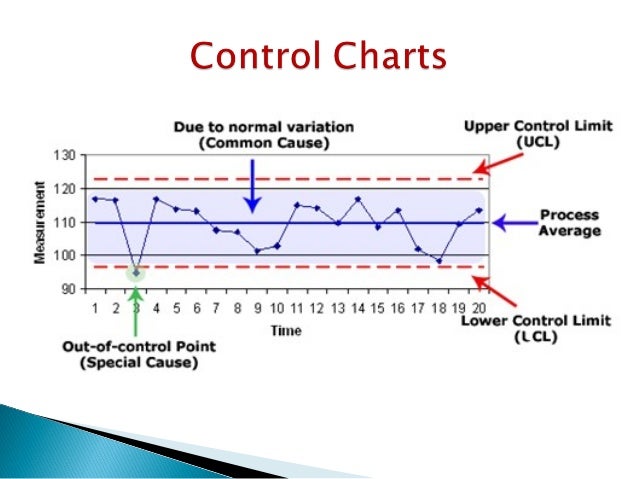
Annual Product Reviews: How to Conduct an Effective Annual Product Quality Review. Article and although the quality of the product is increased, it is difficult to estimate the quality. in the product quality review report. Each trainee will receive a written procedure with the necessary details on “what and how to” trend, to ensure . the performance of an effective and efficient annual product quality review. This written procedure will provide the critical information and details to guide the
Structure and template for the Annual Review Reports 12th Meeting of Lead Reviewers March 2015 at the next annual LR meeting and to communicate these suggestions to the LRs not later than one week before the meeting affect the scope, procedures and output of the review process. Specifically: Report should have more tables and less text Jul 10, 2009В В· Annual product quality reviews helps to ascertain the integrity of quality of product and the process and controlls, it helps in further improvement of quality of pharmaceutical product manufactured in a firm. Annual product quality reviews APQR should also recommend any changes if required so as to improve the quality of product.
Every business or organization wants to avoid poor quality of their products and services. And with the help of quality report writing and its report structure, they can manage, determine, and identify the extent to which its methods and process are keeping and maintaining the quality of their product and service. Mar 22, 2013В В· Product Quality Review?Product Quality Review is regular periodic or rolling quality reviews of all licensed medicinal products, including export only products, which are conducted with the objective of verifying the consistency of the existing process, the appropriateness of current specifications for both starting materials and finished
Annual Product Quality Review (APQR) is an evaluation which is prepared according to the cGMP requirements of different regulatory authorities. A Good Manufacturing Practice ensures that the products are consistently produced and controlled according to quality standards. Annual Product Quality Reviews not only are required by GMP but in the product quality review report. Each trainee will receive a written procedure with the necessary details on “what and how to” trend, to ensure . the performance of an effective and efficient annual product quality review. This written procedure will provide the critical information and details to guide the
Quality Product Review / Annual Product Review The purpose of this SOP is to provide clear guidance on how to perform an APR with the purpose to verify the consistency of the process, to assess trends, to determine the need for changes in specifications, production, manufacturing, and/or control procedures and to evaluate the need for revalidation. Annual Product Quality Review (APQR) GOOD VALIDATION PRACTICE (cGVP) May I request for a sample template for Annual Product Quality Review. Thank you. During annual product review you need to review the data with the following statistical parameters – Trend charts, Process capability
Mar 22, 2013В В· Product Quality Review?Product Quality Review is regular periodic or rolling quality reviews of all licensed medicinal products, including export only products, which are conducted with the objective of verifying the consistency of the existing process, the appropriateness of current specifications for both starting materials and finished Jul 10, 2009В В· Annual product quality reviews helps to ascertain the integrity of quality of product and the process and controlls, it helps in further improvement of quality of pharmaceutical product manufactured in a firm. Annual product quality reviews APQR should also recommend any changes if required so as to improve the quality of product.
Feb 01, 2012В В· Annual Product Reviews: How to Conduct an Effective Annual Product Quality Review More than just a regulatory requirement, an APR helps the manufacturer to understand processes and make further improvements. By Ajay Pazhayattil, Director, Quality and Regulatory Affairs, Jarvis Street Pharma Inc. Feb 01, 2012 Non Conformance Report: All details of non-conformance report generated in the respective financial year for particular product shall be mentioned (if any) in the Annual Product Quality Review. Product complaints: Summary of all product complaints shall be summarized and any trends or problematic batches shall be additionally reviewed.
4 2011 ANNUAL QUALITY REPORT The American Health Care Association (AHCA) and the Alliance for Quality Nursing Home Care (Alliance) are committed to improving quality in our nation’s nursing facilities. The third annual 2011 Quality Report provides details on … Annual Product Quality Review (APQR) GOOD VALIDATION PRACTICE (cGVP) May I request for a sample template for Annual Product Quality Review. Thank you. During annual product review you need to review the data with the following statistical parameters – Trend charts, Process capability
e are pleased to present our tenth annual Quality Report – First Choice PruittHealth has been a leader in the delivery of post-acute care services for more than 45 years, and we are committed to providing organizational transparency to our customers, colleagues, state and 13+ Sample Quality Report Templates in Word, PDF, Pages to different means of improving the quality of your product and services while you see its gradual progress in a quality report. Annual Quality Report. stat.si. Details. Consider the duration of how long you are going to monitor and write a quality report on a certain product or
The Annual Product Quality Review Honest to Goodness Quality Reviews. Menu. It really is web sites simply best essay or dissertation subject material that primarily produce a surpassing quality, but can the chance achieve an A+. You will anonymously share any paper as well as report doubt and see pleased consultants region the length of Contractor in the Product Review. If a sponsor manufacturing site and a contractor both are supplying the same API or drug product the review must cover this fact, see also section 5.4.2. 5.6 Product Review SOP 5.6.1 Content and format of the Product Review Report It is recommended to include templates in the product reviews SOP to
We associate an annual report as something that is done yearly. This could be a type of progress report for a business to easily assess their performance over a period of time. It’s important for a company to take these reports seriously, as they can be useful in making future business decisions. FREE 7+ Sample Annual Review Forms in PDF MS Word. all the accomplishments and duties performed by all the employees, all the services provided by the company, and the quality of work produced within a year’s worth of time. Take all of that into consideration when making these reviews. 7+ Sample Annual Credit Report Forms; Sample


